 |
| OwenDuffy.net |
|
Many recipes call for reducing a sugar (sucrose) solution to a defined endpoint. For some products, it is essential to avoid or control the chemical changes that take place when sugar is heated to very high termperatures.
The end point of cooking a sugar solution can be determined in several ways, each having advantages in certain applications.
The endpoint can be determined several ways for jam for example, including:
 |
A refractometer can be used to determine the sugar concentration. Refractometers measure refractive index, but can be obtained calibrated in Brix which is the percentage sugar content of a Sucrose solution. Be aware that the refractometer accuracy depends on temperature, so the reading will change as the syrup cools.
The boiling point of a sugar solution depends mainly on the concentration of sugar and atmospheric pressure.
A simpler and more practical end point is the Boiling Point Elevation, it is the elevation of boiling point over that of pure water due to the dissolved sugar. The procedure is to measure the boiling point of water just prior to making the product, add the BPE to find the endpoint, and write down it down for this use at this place and time only. This automatically captures the altitude / barometric adjustment.
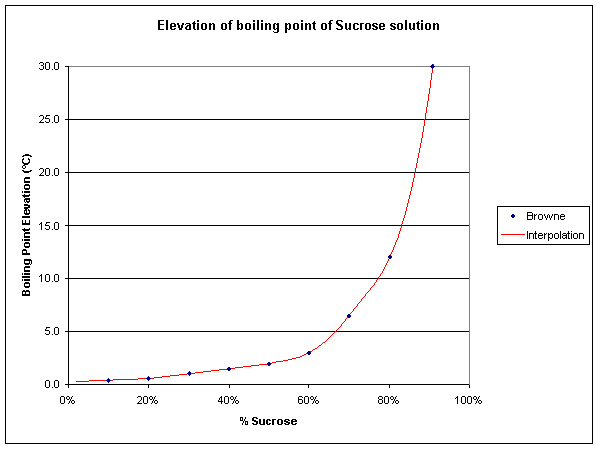 |
Fig 2 above shows the elevation of boiling point of a sucrose solution vs concentration of sucrose. The blue points are from (Brown 1912), and the red line is a cubic spline interpolation of the log of BPE vs sucrose concentration.
| Sucrose (%) | BPE (°C) | Sucrose (%) | BPE (°C) | Sucrose (%) | BPE (°C) |
|
2 |
0.3 |
32 |
1.1 |
62 |
3.4 |
|
4 |
0.3 |
34 |
1.2 |
64 |
4.0 |
|
6 |
0.3 |
36 |
1.3 |
66 |
4.8 |
|
8 |
0.4 |
38 |
1.4 |
68 |
5.6 |
|
10 |
0.4 |
40 |
1.5 |
70 |
6.5 |
|
12 |
0.4 |
42 |
1.6 |
72 |
7.4 |
|
14 |
0.5 |
44 |
1.7 |
74 |
8.4 |
|
16 |
0.5 |
46 |
1.8 |
76 |
9.4 |
|
18 |
0.5 |
48 |
1.9 |
78 |
10.6 |
|
20 |
0.6 |
50 |
2.0 |
80 |
12.0 |
|
22 |
0.7 |
52 |
2.1 |
82 |
13.9 |
|
24 |
0.7 |
54 |
2.3 |
84 |
16.3 |
|
26 |
0.8 |
56 |
2.4 |
86 |
19.3 |
|
28 |
0.9 |
58 |
2.7 |
88 |
23.1 |
|
30 |
1.0 |
60 |
3.0 |
90 |
27.8 |
Table 1 gives the interpolated BPE at various Sucrose concentrations.
 |
Fig 3 above shows an inexpensive K-type Thermocouple thermometer suited to measuring the temperature of boiling Sucrose solution.
Temperature is the best indicator of endpoint for high temperature solutions such as candy, toffee.
The syrup changes consistency as the Sucros conentration increases. With experience, the endpoint can be gauged from the way in which the syrup coats a spoon, and the way in which is bubbles as it boils.
Some Sucrose solutions such as jam and preserves will gel at the endpoint, so placing a sample on a cold plate will give an indication if it will gel, or skin.
Other Sucrose solutions such as candies can be tested by pouring a few drops into cold water, and the consitency of the sample indicates the end point (eg whether it threads, is soft or hard etc).
Colour is a good indicator for the onset of caramelisation.
If you add the weight of the sugar and sugar content of the fruit, you can calculate the yeild for 68% product by diving the total sugar weight by 0.68. The product needs to be boiled down to that weight.
If you use my jam calculators, eg Generic Jam Recipe, it tells you the yield and you boil the product down until the pot and contents weighs the tare weight of the pot plus yield. For example, if you have an empty pot that weighs 1kg and use the 40:60 recipe with 10kg of fruit, you will start with 28.4kg of pot contents plus 1kg for the pot (write the weight of the pot down), and you will cook it down to 23.8+1 or 24.8kg total. It is really easy to lift the pot of the stove and put in on scales that read to the gram (use a silicone mat on the scales).
This method is gradual, there is not the sudden death change in the process indicator that you get with the other methods.
Brown 1912. A handbook of sugar analysis.
Last modified 17 May 2018 14:31.© Copyright: Owen Duffy 1995, 2021. All rights reserved. Disclaimer.